The maturity of 3D printing technology has made a qualitative leap in the medical field. Recently, we have seen the US Food and Drug Administration (FDA) approve several medical products related to 3D printing to enter the market. For example, last week, pharmaceutical company Aprecia used the 3D printed ZipDose tablet for the treatment of epilepsy - SPRITAM levetiracetam was approved by the FDA. And a few weeks ago, two other companies, joimax® and Oxford Performance Materials, also received market access from 3D printed medical implants. It seems that 3D printing is being accepted by the medical regulatory authorities.
Today, the news about Aprecia is gone, and a smaller dental company, DENTCA from Los Angeles, officially received FDA 510(K) approval, and its new 3D printed material can be used as a denture base.

“After several years of development, DENTCA's new 3D printing base is finally ready for use. We are very excited to start applying this technology and continue to drive the revolution in the denture world,†said Dr. Jason Lee, one of DENTCA's technology founders. "This material is a light-curing resin that can be used to make and repair all and part of dentures and dentures; this will eventually replace traditional heat-curing and self-polymerizing dentures. With the help of high-precision 3D printers We can improve the manufacturing process so that the denture manufacturing process is faster, more accurate and more predictable."
This material can be used in an SLA 3D printing process to create a denture base that has passed all required cytotoxic, irritating, genotoxic, sensitizing, acute toxicity, etc., and biocompatibility of material characterization. Comply with FDA Blue Book Memorandum #G95-1 and International Standard ISO 10993-1.
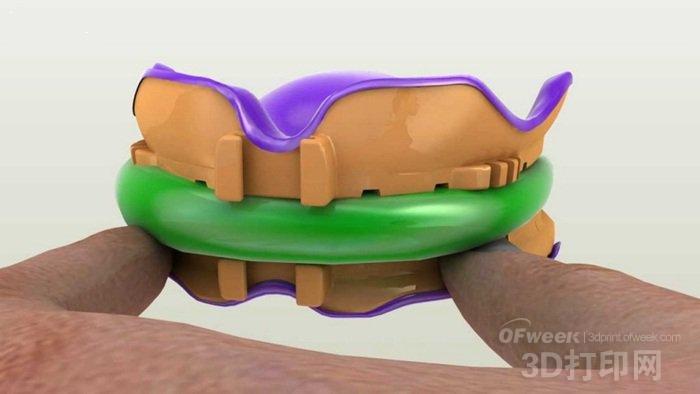
“This approval completely revolutionized the denture manufacturing process, which has remained almost unchanged over the past 100 years,†explains Sun Kwon, CEO of DENTCA. “The new development will bring incredible possibilities to patients, and doctors will soon be able to In their office, 3D prints the final dentures, allowing the manufacturing process to be completed in one day."
The technology behind DENTCA's products enables all dentures to be manufactured 2.5 times faster than traditional methods. DENTCA combines its sophisticated CAD/CAM technology with 3D printing to eliminate human error and greatly reduce the number of visits and visits per patient before receiving a denture. In addition, the manufacturing cycle has been reduced from 30 days to only 5 days.
Socket Head Cap Screws,Hexagon Socket Head Cap Screw,Hexagon Socket Round Head Screw,Phillips Hex Head Screw
we enhanced the hardness of the screws by heat treatment which can efficiently prevent slip and break. With a stable and reliable quality, Lanejoy screws will be your best choice.
China Socket Head Cap Screws,Hexagon Socket Head Cap Screw,Hexagon Socket Round Head Screw,Phillips Hex Head Screw, we offered that you can trust. Welcome to do business with us.
Machine screw,tapping screw,self drilling screw,flat head screw,Pan head screw
Shenzhen Lanejoy Technology Co.,LTD , https://www.szsmallcompressionspring.com