Abstract: This article describes the chemical principle, raw materials and equipment of UV curing adhesives. The working principle and characteristics of photoinitiators, monomers, prepolymers and main additives were discussed. At the same time, it briefly introduced the status quo and development in this field.
Keywords: UV curing, adhesive
Foreword
Ultraviolet (UV) and electron beam (EB) curing belong to the category of radiation curing, and they are characterized by fastness, simplicity, environmental protection, and high efficiency. Among them, UV curing technology has been widely used in the paint and printing industry. In recent years, the application of UV curing technology in adhesives has also received increasing attention.
UV-cure adhesives have the following characteristics: 1. Fast curing at room temperature, curing time can be controlled from a few seconds to a dozen points, 2. The curing process is easy to operate and control, can be partially cured, 3, single component solvent-free, to meet environmental protection requirements . Therefore, although the development of UV-cured adhesives started later than other adhesives, there has been an increasing trend in the applications of the electronics industry, communications and information industry, optical instrument manufacturing and technology products. Especially in developed countries, the efficient and automated production process and the practicalization of a variety of miniaturized UV-curing devices have led to the development and application of this type of adhesive.
UV-curable adhesives can be divided into free radical polymerization (such as acrylates and unsaturated polyesters) and ionic polymerization (such as epoxy resins) according to the principle of curing reaction. Among them, acrylate UV curing adhesives are the most widely used. Known as the third generation of acrylic adhesive, this article briefly introduces the principle and development of this type of adhesive.
1 Basics of UV Curing Adhesives
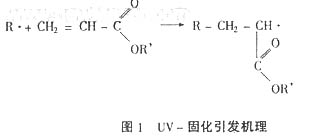
1.1 Curing principle
In UV curable adhesives, the photoinitiator is exposed to ultraviolet light and reaches an excited state, and active radicals are generated by cleavage or hydrogen transfer of the initiator molecules. These radical-initiated olefinic bonds in the acrylates produce new free radicals (Figure 1), resulting in free radical chain polymerization of the entire system.
1.2 Raw materials and equipment
UV-curing adhesives generally consist of a light-curing prepolymer (25-90% by weight), a monomer (15-60%), a photoinitiator (1-3%) and other additives, and sometimes a sensitizer and a photoinitiator. To increase curing efficiency. This adhesive quickly and simply cures under ultraviolet light.
1.2.1 Photoinitiator
There are many types of UV photoinitiators. The widely used and effective ones are aromatic carbonyl compounds, mainly phenyl ketone compounds such as dibenzoyl, benzophenone, benzoin and their ether derivatives. According to the mechanism of free radical generation, these initiators can be divided into two types: split type and hydrogen transfer type (Figure 2). Especially the cracking type is more commonly used.

The decomposition of benzoin generates two free radicals, and the two generated primary radicals can initiate monomer polymerization. However, if primary radicals diffuse too slowly, they will cause coupling and reduce the efficiency of initiation. Benzoin has strong intramolecular hydrogen bonds. The results of the study showed that etherification of the hydroxy groups of benzoin can destroy the intramolecular hydrogen bonds and increase the photolysis rate. For example, benzoin methyl ether tends to increase the photo-curing rate by about 2 times over benzoin. It should be pointed out that benzoin methyl ether is poor in storage stability, so it is desirable to obtain good activity and long service life, with benzoin isopropyl ether being preferred.
(to be continued)
Heat Transfer Label,Private Label,Clothing Labels
Ningbo Leya Printing Co., Ltd , http://www.leya-thermaltransfer.com